What is dehydrogenation of alcohol reactions in organic chemistry?
Dehydrogenation is an important chemical reaction, usually associated with the removal of hydrogen from organic molecules. It is the opposite reaction of hydrogenation.
Dehydrogenation reactions produce aldehydes, ketones, alkenes, alkynes, polymers and aromaticand aliphatic closed chain compounds.

The reaction takes place in the presence of a catalyst at a temperature of 300–500 ᵒC. Dehydrogenation of aromatics and aliphatic alcohols are mainly discussed here basically.
Dehydrogenation of alcohol reactions examples.
When alcohol vapor is passed over a heated metallic copper catalyst at a temperature of 300 ᵒC, the alcohol is oxidized to produce aldehydes and ketones.

Aldehydes are produced when the primary alcohol is oxidized and ketones are produced when the secondary alcohol is oxidized.
In this case no oxidant is used. Therefore the aldehydes and ketones produced cannot be re-oxidized and converted into carboxylic acids
For example, formaldehyde is produced when methanol vapor is quantitatively mixed with air or oxygen and heated at 250 ᵒC in the presence of a catalyst.
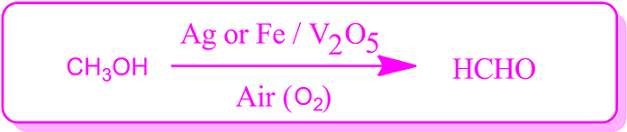
A mixture of silver powder and V2O5 is used as a catalyst in the reaction.
It is the dehydrogenation reaction of methanol, by which formaldehyde is produced industrially.
Why does 3ᵒ or tertiary alcohol not participate in dehydrogenation reaction?
Tertiary or 3ᵒ alcohol does not participate in the dehydrogenation reaction as no hydrogen is added directly to the carbinol-carbon of alcohol. In this case alkene is produced by the dehydration reaction.

What is iodo-form test for ethanol in organic chemistry?
Compounds that contain keto-methyl group or compounds those are oxidized during reaction to form keto-methyl group containing compounds, react with iodine in the presence of alkali to form iodo-forms.

This reaction is called iodo-form test. For example, ethanol is oxidized to acetaldehyde during reactions, in which the keto methyl group is present. Hence, ethanol responds to iodo-form tests.
