Phenol definition-Phenol structure and Identification in chemistry
The hydroxyl derivatives of aromatic hydrocarbon which contain hydroxyl group are known as phenols .
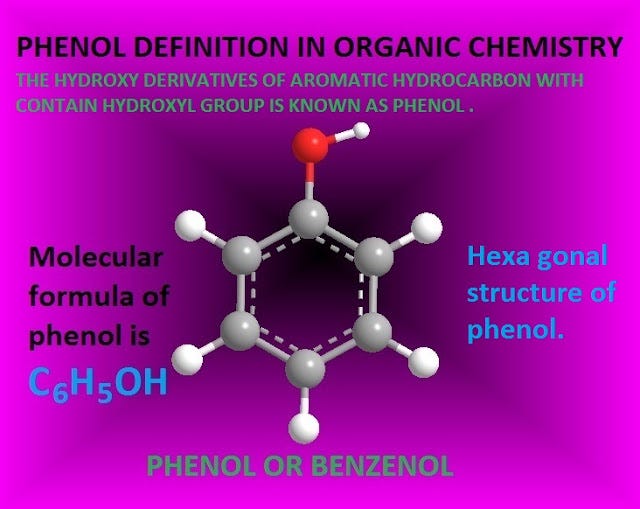
Phenol is an organic as well as aromatic compound with molecular formula C6H5OH .
Phenol is also known as benzenol, carbolic acid, or as hydroxylbenzene. It is white or colourless crystalline and also a volatile solid compound.
Phenol was first extracted from coal tar . But now a days, phenol is produced in a large scale from petroleum .
Phenol structure and molecular formula
Phenol is an organic as well as aromatic compound with molecular formula C6H5OH.
That is it consists of one phenyl group ( — C6H5 ) and one hydroxyl group ( — OH ), attached with phenyl carbon atom.
Each carbon atom of phenol is sp2 hybridized. The oxygen atom of phenolcontains two lone pair of electrons.
The ‘O’-atom shows sp3 hybridization. Phenol has hexagonal planar structure .

The hexagonal planar structure and chemical or molecular formula of phenol is shown below.
Phenol resonating structure
The lone pair on oxygen atom of O–H bond in phenol participate in resonance with the pi electron of benzene ring .
There are five resonating structure of phenol through which phenol gets stability .
The resonating structures are ,

Identification of phenol with ferric chloride .
In addition of few drops of neutral ferric chloride ( FeCl3 ) solution to phenol , the entire solution becomes violet in color .
Because in neutral solution,phenolreacts with ferric chloride ( FeCl3) to form violet color complex ion [ Fe ( OC6H5 )6 ]3-
Hence the color of the solution becomes violet. Phenol is identified with the help of this reaction .

Indeed, all the aliphatic or aromatic compounds with enol group create a characteristic color with neutral ferric chloride solution .
Depending on the structural shape of phenol the color of the solution may be green, blue, even red including violet.
Identification of phenol
When phenol is dissolved in concentrated sulfuric acid and a few drops of aqueous sodium nitrite is added ,the color of entire solution change into red on dilution.
Finally the color turns blue when made alkaline with aqueous sodium hydroxide.

With the help of the above experiment phenol is identified . The above experiment is known as Liebermann reaction .
Why o-nitro phenol is more acidic than o-methoxy phenol ?
In case of o-nitro phenol, the compound contains strong electron withdrawing nitro group( –NO2 ). Due to –R and –I effect of nitro group , the density of electron on phenolic oxygen atom decreases.
As a result , the ‘O’-atom attract the O — H bonded electron towards itself . Hence the O–H bond becomes more polar as well as weak .

Consequently, the weak O–H bond dissociate easily to leave proton in aqueous solution and thus o-nitro phenol shows acidic nature .
On the other hand , in o-methoxy phenol, due to +R effect of electron repulsive –OCH3 group , the density of electron on oxygen atom as well as ‘O’-linked carbon atom increases.

As a result, the displacement of O–H bonded electron towards ‘O’-atom does not happen . Hence , the O–H bond becomes less polar that is more stronger with compare to O–H bond of o-nitro phenol.
Therefore ,O–H bond does not break easily . Hence o-methoxy phenol shows less acidic property with compare to o-nitro phenol.
