Magnesium oxide balanced equation in chemistry for class 9
There are some magnesium oxide balanced equations in chemistry. Such as magnesium oxide balanced equation between magnesium and oxygen.
Again, magnesium oxide balanced equation when MgO reacts with of sulfuric acid and nitric acid individually.
One example of magnesium oxide balanced equation between magnesium and oxygen is discussed below.
When magnesium ribbon is burned in the air in the presence of oxygen, magnesium oxide is produced.
Combustion of magnesium ribbon is a chemical change. This is because the metal magnesium combines chemically with oxygen to produce magnesium oxide. Any chemical change is just a chemical reaction.
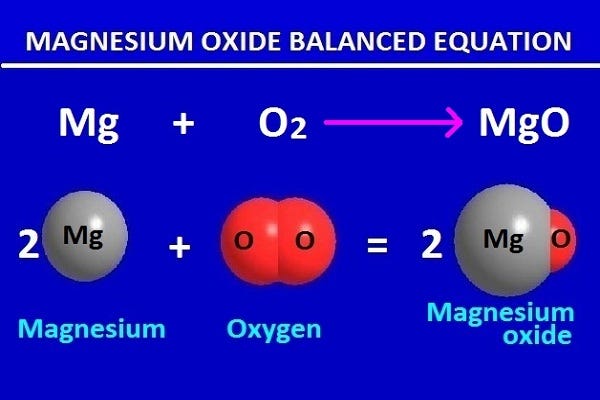
In this chemical changes magnesium and oxygen are reactants and the resultant magnesium oxide is product. These reactants and products are expressed through an equation.
In the above said chemical equation is expressed by writing the reactants on the left and the products on the right. An arrow is drawn from the reactant to the product.
Now, according to the law mass action, the total mass of magnesium and oxygen before the reaction is equal to the total mass of magnesium oxide after the reaction.
Because mass cannot be created nor destroyed. Thus the equation of the magnesium oxide should be balance, so that the number of atoms of magnesium and oxygen on both sides is equal.
Now to equalize the number of atoms on both sides of the equation, we need to multiply both sides with the required number.
When the number of atoms on both sides is equal, the chemical equation is balanced by putting equal marks instead of arrow marks. Therefore the magnesium oxide balanced equation is as follows.

Similarly, the chemical equation of the reaction of sulfuric acid and nitric acid individually with magnesium oxide is balanced.
The magnesium oxide balanced equation with sulfuric acid and nitric acid are shown below.
Magnesium oxide and nitric acid balanced equation

Magnesium oxide and sulfuric acid balanced equation


