Halogen family elements-properties-periodic table-oxyacids-radioactivity.
Halogen family elements
According to modern periodic table , group 17th consist of five elements , namely , fluorine, chlorine, bromine ,iodine, astatine and tennessine( Ts ) which do not occur free in the nature, are collectively known as halogen family.
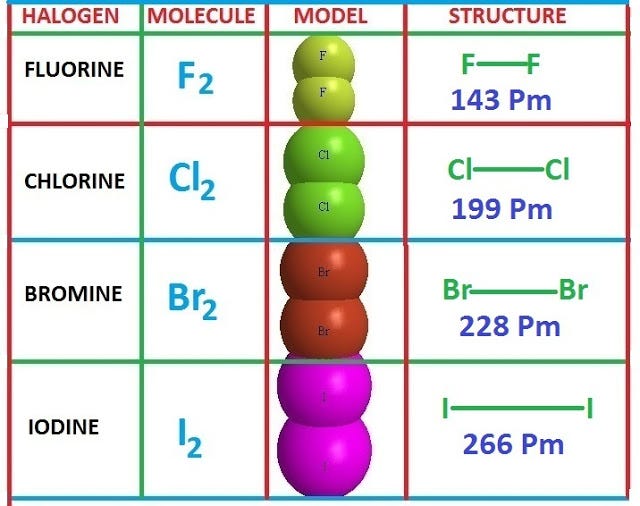
Indeed, fluorine, chlorine, bromine and iodine have closely related properties and are known collectively as the halogen family elements.
Halogen family elements properties
General electronic configuration of halogen elements are, ns2,np5. That is the outer most shell of halogen atom contains seven electrons .
Hence halogen element have tendency to gain one more electron to complete their octet .
Consequently, halogen elements are non metal as well as strong electronegative .The electronegativity of halogen elements decrease as we move from top to bottom along a group .
Halogen elements have high affinity for electron, that is they have a strong tendency to pick up electrons . For this reason, they exhibits strong oxidizing power.
For example, chlorine oxidized metallic sodium and non metallic nitrogen elements to form sodium chloride and nitrogen trichloride respectively.
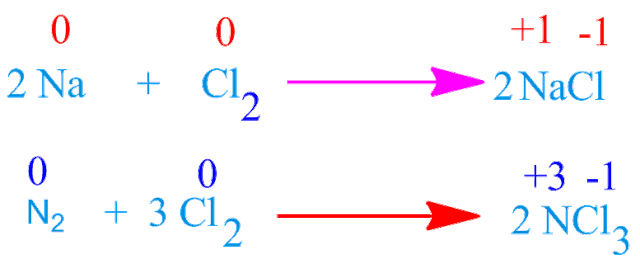
They can oxidize metal as well as non metal . Although ,the oxidizing power decrease on moving down the group .
Halogen family elements periodic table
According to modern periodic table , group 17th consist of five elements , namely , fluorine, chlorine, bromine ,iodine and astatine which do not occur free in the nature , are collectively known as halogen family elements .
Indeed , fluorine, chlorine, bromine and iodine have closely related properties and are known collectively as the halogen family elements.
Radioactive halogen family elements
Among the halogen family elements, fluorine, iodine and astatine have their radioactive isotope in nature.Fluorine has one stable natural occurring isotope, F19.
However, there are trace amounts of radioactive isotope of fluorine is found in nature via cluster decay of Pa231.The radioactive isotope of fluorine is F23.
There are no remarkable radioactive isotope of chlorine and bromine are found in nature .
But ,there are trace amounts of radioactive isotope of iodine is, I 129 ,which occurs via spallation and from the radioactive decay of uranium in ores.
Several other radioactive isotopes of iodine have also been created naturally through the decay of uranium .
There are four naturally occurring radioactive isotopes of astatine which are produced via radioactive decay of uranium, neptunium, and plutonium.
These radioactive isotopes are At 215 , At 217, At 218 and At 219 . The last elements of this group is tennessine(Ts),which have two synthetic radioisotopes, Ts 293 and Ts 294.
Oxyacids of halogen family elements
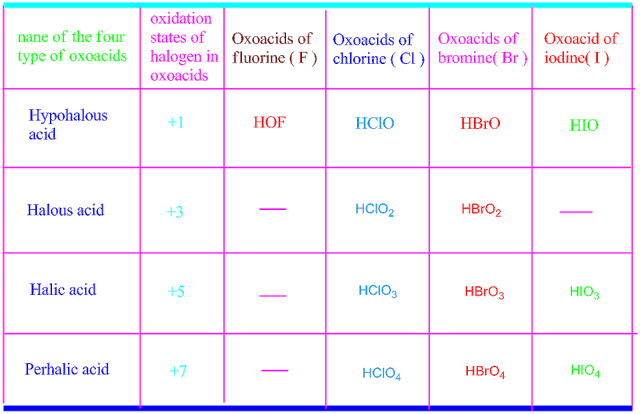
Fluorine does not form oxy acids .This is because , fluorine is more electronegative than oxygen. But chlorine,bromine and iodine can form oxy acids with oxygen.
They usually form four types of oxy acids , namely, hypohalous acids , halous acids , halic acids and perhalic acids .
The acidic strength and stability of all the four types of oxy acids are shown in the following table .
Why fluorine does not form oxyacids ?
In oxy acids , oxygen shows negative oxidation states . Hence , halogen elements which are less electronegative than oxygen, are form oxy acids via oxidation by oxygen atom. Now, fluorine is more electronegative than oxygen .
The electronegativity pauling scale of fluorine is 4 where as that of oxygen is 3.5. That is fluorine can not oxidize by oxygen atom.
Consequently, fluorine does not form oxy acids with oxygen molecule. Only one known oxoacid of fluorine is hypofluorous acid, with chemical formula HOF.
Article summary
